This originally appeared in the July/August issue of Discover magazine as “Fire in the Belly” Support our science journalism by becoming a subscriber.
How’s your tummy feeling today? Odds are, not that great. A new study estimates that Americans spent $135.9 billion on treatment for gastrointestinal diseases in 2015, over the course of more than 54.4 million trips to the doctor. Irritable bowel syndrome (IBS) alone is thought to affect 10 to 15 percent of all people worldwide, many of whom are undiagnosed.
Your digestive system achieves no small feat each and every day, as it turns things you eat into usable energy and nutrients for the rest of your body. It’s an incredibly complex system, which also means there’s incredible complexity in the ways things can get out of whack.
But while gut issues are on the rise, so is our understanding of them. Researchers have made huge strides toward figuring out the causes of — and solutions for — some of the most common culprits behind your stomachache. And most of this progress is thanks to new mechanistic understandings of how the bacteria in your gut really operate.
We’re All in This Together
It seems like the cutting edge of nearly every corner of the health field — from eczema to Parkinson’s disease — is finding new links to the gut microbiome. This community of around 38 trillion bacteria that live mostly in your colon helps digest your food and protects your body from intruders hitchhiking on your last snack. And it turns out that these processes in your gut can affect the whole body, thanks to the downstream effects of inflammation, nutrient absorption and the compounds that bacteria produce.
The gut microbiome is quite diverse: No two people have exactly the same bacteria species in exactly the same quantities, and the bacteria themselves can have huge diversity in their genes. This variation adds an extra challenge to research, since lessons learned from one person’s gut might not apply to someone else’s.
Nevertheless, researchers have already found links between the gut microbiome and some of the most common diseases of the digestive system. A 2017 study in Science Translational Medicine found that transplanting fecal microbes from patients with IBS into healthy mice led to an increase in adverse gut symptoms in the animals. And a 2018 study in the same journal found clear differences in the microbial community between people with IBS, inflammatory bowel disease (IBD) and healthy individuals — differences that were clear enough that they could soon be used to diagnose the two gut conditions.

A healthy intestinal tract contains villi, which help absorb nutrients. Inflammation can wreak havoc on intestinal cellular walls. (Credit: NoBeastSoFierce/Shutterstock)
Some researchers remain skeptical, though, that these microbes are the source of our problems and not just a byproduct of poor health. For instance, a 2019 review in Gastroenterology looked at 24 different studies from the previous nine years and found consistent patterns in the gut microbes of IBS patients, but no evidence the microbes were causing symptoms.
But others are learning more and more about the actual mechanisms for how microbes are affecting our guts — and controlling whether we’re sick or healthy.
Who’s the Boss?
Though the field of gut health is still in its infancy, preliminary findings are interesting and encouraging. For instance, in a 2016 study in Nature Genetics, a team of Dutch researchers looked at people who have genes that lower their production of lactase — the enzyme that digests lactose sugars found in dairy products — but still eat dairy products. They found that these people were likely to have a greater abundance of Bifidobacterium, a type of gut bacteria that digests lactose sugars. A team of Japanese researchers confirmed the finding in a 2018 study in PLOS ONE. In other words, some people’s gut microbes could actually make up for something they’re lacking in their genes.
“It hasn’t been robustly verified; it’s just a tantalizing correlation,” says Mary Kable, a research molecular biologist at the USDA Agricultural Research Service in Davis, California. “But that’s the kind of information that’s out there. There are a lot of associations that haven’t really all been followed up on yet.”
Another promising avenue is looking not at the identities of gut bacteria themselves, but studying the compounds that the bacteria produce, called metabolites. For instance, in a 2019 study in Cell Host & Microbe, researchers found that IBD patients have a particular strain of Bacteroides in their guts that doesn’t produce as many metabolites called sphingolipids, which regulate inflammation. Researchers confirmed through testing in mice that guts with these sphingolipid-deficient bacteria are more inflamed.
These sorts of discoveries could lead to treatments, says Ramnik Xavier, a clinical gastroenterologist at Massachusetts General Hospital and molecular biologist at the Broad Institute, who led the study. “If you can replace the missing metabolite, then you could also promote healing,” says Xavier. “Microbiome science for a long time was very descriptive. But now it’s moving to the phase of mechanism and function. And once we have those pieces in place, then we can start thinking about diagnostics and therapeutics.”
Inflammation Nation
IBD and other gut conditions like celiac disease, as well as systemic diseases like heart disease and diabetes, all have links to the body’s inflammatory response. Inflammation may be a dirty word these days, but it’s actually a normal, adaptive part of your immune system. It’s what makes the skin around a papercut turn a little pink, and what makes a sprained ankle warm to the touch. The damaged cells spit out chemicals that increase blood flow to the area and call white blood cells in to attack intruders to help with healing.
But when bodies aren’t functioning properly, unnecessary inflammation can become a burden. Some people’s immune systems might think something is an invader that isn’t. In celiac disease, for example, the body wages war on gluten molecules. In ulcerative colitis and Crohn’s disease — the two main kinds of IBD — the body attacks its own gastrointestinal tract. And with true food allergies, like some people have to peanuts or shellfish, the immune response can be so strong that it triggers anaphylaxis, a bodywide shock that can be life threatening.
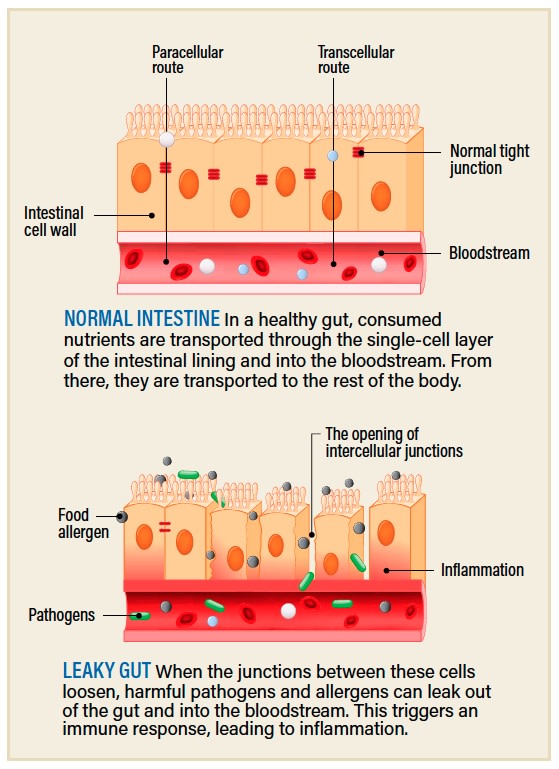
(Credit: Designua/Shutterstock)
Other causes of chronic inflammation are even less straightforward. Sometimes a combination of illness, stress or poor diet can lead to an increase in the permeability of a person’s intestines, a condition colloquially referred to as leaky gut. In healthy intestines, a layer of protective mucus keeps food, bacteria and whatever else might be passing through your gut firmly inside the tube and away from your internal organs. But if this barrier weakens or is damaged, the contents of the gut, including bacteria, begin to escape, which triggers a substantial immune response. If this happens repeatedly, you can end up with chronic inflammation that can lead to downstream effects, from neurodegenerative diseases to cancers.
Read more: How COVID-19 Affects the Gut
Leaky gut has historically been poorly understood. But earlier this year, researchers from the University of California, San Diego, grew tiny pieces, or organoids, of human gut in the lab to better understand the condition. They were able to turn healthy guts “leaky” by exposing the organoids to certain bacteria, which stressed them out until the junctions between cells began to loosen and become permeable, leading to telltale signs of inflammation. And they even identified a drug that seemed to reverse the effects and retighten the junctions: the diabetes drug metformin.
But what causes a gut to leak in the first place? That’s not as well understood. A team in the U.K. is following up on one hypothesis that’s had some preliminary support: Emulsifiers, a common food additive in processed foods, may have a negative effect on our gut health.
Guts, Emulsified
Emulsifiers are everywhere in processed foods. They’re a type of compound that helps fats mix with liquids: They’re the reason your mayonnaise, ice cream and peanut butter don’t separate. A handful of studies done by a research group at Georgia State University have found that emulsifiers in the diet alter the composition of gut microbes in mice, leading to chronic inflammation and even behavioral changes.
“The actual definite mechanism for how [emulsifiers] act within the gut isn’t particularly clear at the moment,” explains Dom Partridge, a research fellow at the University of Aberdeen. Partridge says the current working hypotheses are that emulsifiers, which are also called surfactants or detergents, act to make liquids slipperier. Just as they act on liquids outside of the body, like in food, they could act on liquids inside the body, like in your gut, altering the viscosity of the mucosal lining in your intestines. This type of change in the gut environment could in turn affect which gut bacteria can live there.
But so far, evidence of the effects of emulsifiers has only been found in mice, rats and cells. “And there are huge differences between mice and humans,” says Partridge. That’s why he and his research group have launched a study to investigate their effects in humans. They gave a group of participants a controlled diet for four weeks, first with the addition of the common emulsifier soy lecithin, then again without, to see how their gut microbes and overall health changed. The samples are still being analyzed.
Partridge, however, isn’t convinced they’ll find evidence that the common food additives are that harmful to humans. “I’ll be potentially eating my words when we see the data,” he says. “But as it stands, although there’s a lot of info about emulsifiers in gut health in animal work, I am very skeptical that this is going to translate into any form of negative impact on humans.”
Part of the reason for this, he says, is because of how hard it is to study something that might have chronic, rather than instantaneous, effects. He uses the link between saturated fat and cardiovascular disease as an example: “This is something that’s taken years to reach any kind of real consensus on. It’s not something that a few studies have been able to show conclusively. It’s something that’s required input from animal work, from cell work, from human clinical studies to epidemiology. And it’s required all of these types of studies to demonstrate an adverse effect.”
On top of that, proving that a gut is “leaking” is still a major challenge. The best way to detect it, researchers think, would be to look for trace amounts of bacteria in the bloodstream. But at levels that low, it’s difficult to differentiate real bacteria results from trace amounts of contamination that might be in materials used in the lab. Part of Partridge’s work is to develop a method that will reduce the risk of this contamination. “I’m still working on it,” he says.
You Are What You Eat
Emulsifiers are just one example of the many foods, food additives and other consumables that researchers are testing for effects on the gut. There’s already strong evidence that diet, in a general sense, can have major effects on gut microbes, and, therefore, health. But we’re a long way from proving how specific foods, through their impacts on gut microbes, can have specific effects on health.
Perhaps the one exception is dietary fiber, which researchers say is as close as any to reaching consensus. “Things like different types of fiber, and the impact fiber has — not just on gut health, but on systemic health — I think that that’s probably got the most evidence for it,” says Partridge.
“If you consume dietary fiber, typically you will see an increase in some microbes that can ferment fiber, like Bifidobacterium and Ruminococcus, and you will often also see an increase in short-chain fatty acids,” the metabolites that those microbes produce, says Kable. “I would say that’s about where the consensus in the field stops.”
Even foods as mainstream as probiotics — foods and dietary supplements that, in theory, add beneficial microbes to your gut — aren’t yet fully supported, despite their prevalence in stores and in discussions online. A 2018 study in Cell found evidence that some people’s guts are resistant to colonization by probiotics, meaning that even if they work for some people, they might not work for everyone.
Researchers have recently found support for using what’s called a low-FODMAP diet to alleviate gastrointestinal symptoms. FODMAPs, short for fermentable oligosaccharides, disaccharides, monosaccharides and polyols, are a diverse group of carbohydrates that can be hard for guts to digest — and they’re found in all types of food, from artichokes to apples, wheat to milk, and onions to almonds. Over the past decade, researchers have found that a diet low in FODMAPs can reduce symptoms of IBS, and, more recently, researchers have found it’s also safe and effective for those with IBD.
Precision Nutrition
Perhaps part of the reason for the low-FODMAP diet’s success is that it can be personalized. After a patient cuts all or most of the FODMAP-containing foods from their diets, and sees a consistent reduction in their symptoms, the next step is to reintroduce foods one at a time, identifying which ones are the triggers. So far, these person-to-person differences are something scientists and doctors haven’t been able to explain mechanistically, despite the evidence from studies that the differences are there.
Humans have around 23,000 different genes, but the bacteria that live in humans, as a group, have around 3 million. This means that, beyond the differences in human genes and gut bacteria in each person, the genes within the bacteria might be the most important source of variation of all.
That said, sometimes more than one gene that might be found in more than one type of bacteria can have the same function — that is, produce the same metabolite — in the gut. That means to really understand the effects of the gut microbiome, you don’t just need to identify bacteria; you need to know what they’re up to. For this reason, Kable’s lab group at the USDA in California is turning to a method called transcriptomics, which, instead of identifying bacteria, identifies the bacteria’s active genes. She’s using this information as part of her big-picture approach to studying how healthy people can stay healthy and prevent disease. The end goal: personalized nutrition plans.
“Personalized or precision nutrition is, in the most basic sense, looking for biomarkers or predictors of health outcomes — including the microbiome, genetics, epigenetics and various other lifestyle factors like exercise — and incorporating those all together into some sort of machine learning or algorithm that will predict outcomes,” explains Riley Hughes, nutritional biologist at the University of California, Davis, who works with Kable.
“We’re going to need to bring in as many researchers from different areas as possible to make sure that we’re taking into account all of these different factors that can influence health,” says Hughes.
It’s a big ask, but it’s important, says Kable, because we’re learning that the same nutrition guidelines won’t work for everyone. Her hope is to “actually give a set of [dietary] guidelines for different parameters based on something that you can easily assess about yourself,” she says.
“I don’t know what that would look like yet, because I think we’re a really, really long way from that,” Kable adds. “But that is my dream.”
Journey Through the Digestive System
Seeing, smelling, or even just thinking about food gets your gastric juices flowing. Here’s a look at the trip your food takes through your digestive system.

(Credit: Daniela Barreto/Shutterstock)
Spit Take: Saliva is the first of many digestive juices that your meal will be up against. It moistens the chewed-up food with mucus and water to make it easier to taste and swallow, plus it adds an enzyme to the mix that starts to break down starches.
And, Swallow? Your tongue then pushes a wad of food, called a bolus, into your throat (or pharynx) when you swallow. Your epiglottis ensures the bolus doesn’t go “down the wrong pipe.”
Down the Tube: Once in the esophagus, the food is squeezed downward by a series of coordinated muscle contractions. At the end of this 10-inch journey, it meets its first sphincter and is released into the stomach.
Yummy in My Tummy: Once inside our gutsy sac of juice, our food is smashed into even more of a pulp by the stomach’s muscles, all while being soaked in a mix of hydrochloric acid and digestive enzymes. It’s then sent on its way, a little at a time, into your small intestine.
Call in the Big Guns: Lining the 22 feet of small intestine, exocrine cells secrete mucus and more digestive enzymes, while enteroendocrine cells secrete hormones that trigger the liver, pancreas and gallbladder to fire up and add more digestive juices into the mix. These enteroendocrine cells also “speak” directly to nerves, giving them a direct line of communication with the brain.
Team Effort: Mediating all of this cellular gut activity are our gut microbes. There are about 100 billion bacteria in the small intestine.
1, 2, 3, Absorb! The proteins, fats, carbohydrates, vitamins and minerals your body needs to function are absorbed here. After being digested into the smallest molecules possible, the nutrients are transported into the cells lining the intestines and shipped off into the bloodstream to be taken all over the body, as needed. The interior of the small intestine is full of folds and fingerlike tissues that increase the surface area of these absorptive cells dramatically.
Guardians of the Body All the while, your immune system is on standby, at the ready to pounce if any foreign invaders are identified in what you ingested.
The End of the Line: The digested food then makes its way into the colon, or large intestine. Here, excess water is absorbed, and a new gang of microbes ferments the food that wasn’t digestible. It might cause a little gas. But in general, the food has made its way into poop, and it’s ready to collect in the rectum until it’s potty time.
Anna Funk is associate editor of Discover.