What Is An Atom?
Definition Of Atom – basic units of matter, smallest particle of an element that can exist alone or in combination, basic unit of a chemical element, smallest object that retains the properties of a chemical element.
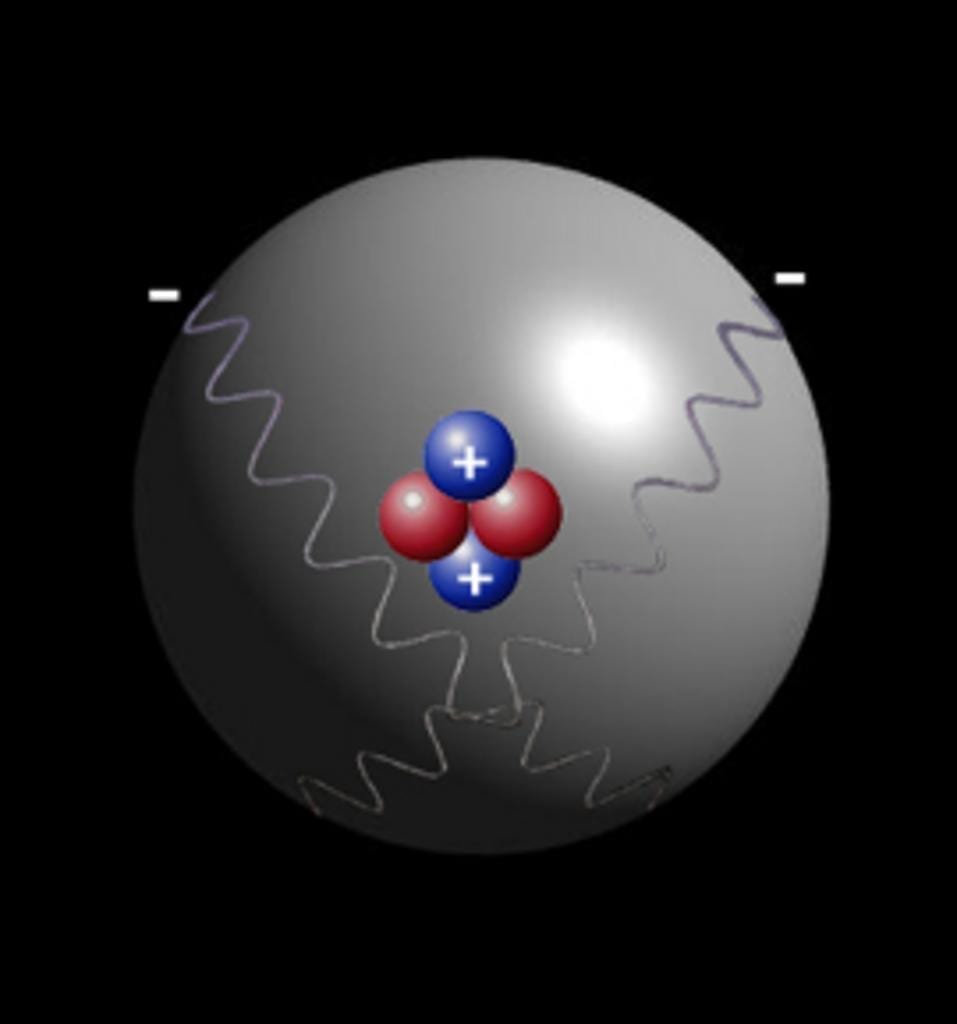
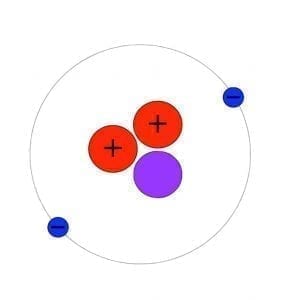
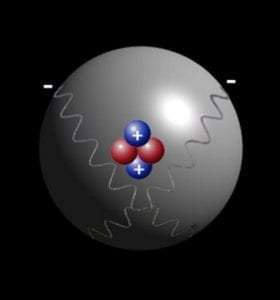
As we all know Atom is smallest particle of an element still having the same chemical properties of the element. There are 2 sextillion atoms of oxygen in one drop of the water. Particles smaller then Atom are called subatomic particles. Electrons, protons and neutrons are the three major subatomic particles. Today scientists understand that protons and neutrons are made of even smaller particles called quarks which are tied together with particles called gluons.
It is believed that an atom is made of 200 or more than 200 subatomic particles because it is told that in an atom there is so much space and electrons take only 1/1000th volume. That space may have more subatomic particles in it and according to the calculations there may come more than 200 subatomic particles. Bellow is the picture of atoms of gold produced by electronic microscope.
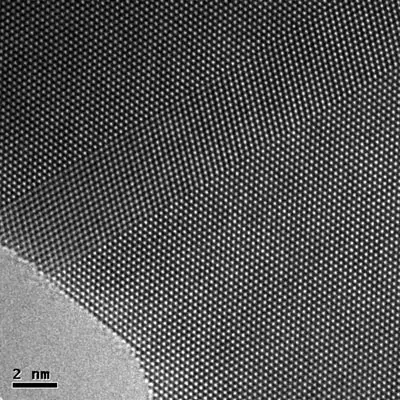
There is always fascinating research taking place with respect to atoms. Researchers and scientists are hard at work looking to make discoveries and draw conclusions that can help society. In August of 2019 researchers revealed a heat shield they created that is only 10 atoms thick.
By definition, subatomic particles are the smallest parts of matter. Every particle in our world is made up of hundreds or thousands of subatomic particles, so it’s almost as if there is no such thing as nothing at all. In a nutshell, the universe consists of countless of particles and each particle is made up of a set of subatomic particles. Without these, we don’t end up with finished things such as pancakes, robots, all the land in Canada, salt, and the ocean.
For example, electrons are one of the elementary particles. These atoms are made up of “gravitons” that affect each other through the electromagnetic field of a super-strong magnetic field. Electrons are often used to measure properties of particles, but at a very large scale, atoms are used to measure atomic masses.
Scientists are still in the process of measuring the sizes of these magnets. However, the size of these magnets has been measured and compared to the size of a proton.
Protons are another subatomic particle. Protons have some magnetic and strong force that keeps them together. The magnetic fields of the protons are what make them so “sticky”. When they come into contact with each other, they will stick together.
The best way to describe the particles of an atom is that they are similar to the size of a magnet. Protons are usually referred to as “fermions”. Scientists use a classification system of an atom to help them determine its chemical makeup. Protons are classified as “fermions”.
Protons have one “charge”, which is equal to one electron. A proton is generally classified as a neutron because they are the same size and make up the same mass. A neutron has two charges, one positive and one negative, and is more powerful than a proton.
Protons are very important in magnetism. It is believed that when you magnetize metal, it turns from a solid to a liquid, and the liquid turns into a gas. Electrons and protons are what is found inside of magnets.
Neutrons are the most complicated subatomic particles. They may be the smallest particles, but they are also the most volatile, unstable, and most of all, the most volatile and unstable particle of all.
Nature Facts: https://www.interestingfacts.org/category/nature-facts
World Facts: https://www.interestingfacts.org/category/world-facts
Science Facts: https://www.interestingfacts.org/category/science-facts
Definitions: https://www.interestingfacts.org/category/definition
Planets: https://www.interestingfacts.org/category/facts-about-planets
Nutrition Facts: https://www.interestingfacts.org/category/nutrition-facts
Information: https://www.google.com
Things To Do: https://www.seatsforeveryone.com/blog
Sitemap: https://www.interestingfacts.org/sitemap.xml
Facts About Atoms
Oxygen has a greater electronegativity. Liquid Oxygen is extremely paramagnetic! Oxidation of BCAAs may raise fatty acid oxidation and play a function in obesity. Facilitated diffusion is another sort of passive transport that makes it possible for things to cross the cell membrane. Have electrons orbit around the exterior of the nucleus. To fully grasp why you can never touch anything, you have to comprehend how electrons function, and before it’s possible to understand that, you should know basic details about the structure of atoms. For the reason, the neutrons to be utilized in nuclear reactions are released by artificial ways. Oxygen along with hydrogen of course make up water.
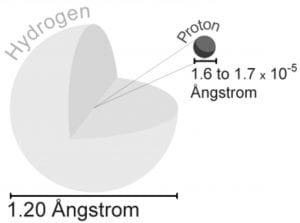
Step 13 After the page was read, review the pieces of the atom again. The same as people, the atoms would like to be complete. It produces light of certain wavelengths. TLW label the kinds of charges the several components of the atom have. For starters, the vast majority of the mass an atom has is concentrated into an unbelievably modest region known as the nucleus. If it had the size of the earth, the nucleus would have the size of a football stadium. Whatever you can see, touch, and feel is composed of atoms the infinitesimally compact constituent parts of matter.
In the event you should examine a slice of iron that isn’t magnetized, you’ll find that the domains within the iron won’t be pointing in the exact direction, but will be pointing in a lot of random directions. Taking well-organized notes can help you comprehend the material. For instance, when you forego a deep layer of fear, you don’t feel afraid, you truly feel relieved of fear.
(Chemistry) Why do the orbitals of an atom only hold a certain amount of electrons? from askscience