Kudos to InterestingEngineering.com and great work by them putting together these interesting and fun facts. This will help you organize all the information along with atoms, chromosomes, RNA, DNA, the atmosphere, water, and basically how stuff fits together in the galaxy and life.
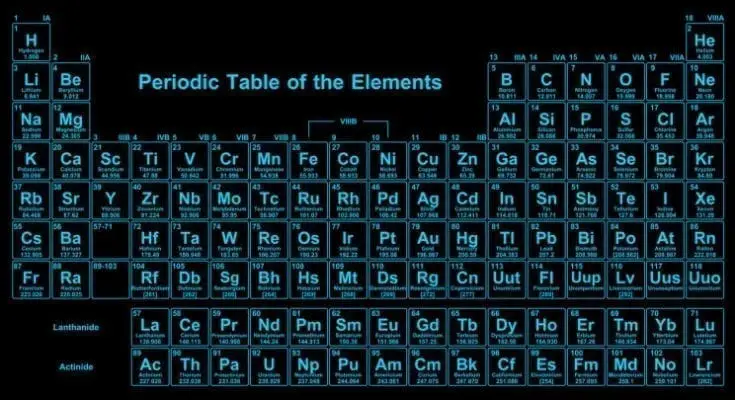
Sadly, to many people, the periodic table is that thing they’re told to monotonously recite in class. But the truth is that this little table is an indispensable roadmap for scientists the world over. Here are a few fun, interesting, and also little-known facts about the periodic table, written […]
Click here to view original web page at interestingengineering.com
Just about every material out there has a symbol that corresponds to its atomic number, and the periodic table of elements is no different. The arrangement of elements in order of decreasing atomic number is known as the periodic table. It is called such because it was created by a man named Alexander von Humboldt who wanted to represent chemical elements based on their shapes, structure, and arrangement in nature.
A lot of work went into creating the periodic table of elements and the purpose of Humboldt was to discover the structure of a particular element so that he could help his fellow scientists work with it. In the process of creating the table, he had to make a decision about what to call each of the elements. He considered naming them based on their electron configuration, but he couldn’t figure out a name for all the elements. Since some of the elements didn’t have clear shapes or configurations, Humboldt decided to try naming them based on their properties.
After a discussion with fellow scientists in a high pressure laboratory setting, he named all the elements using the properties that they each had to offer. Each property of the elements had to offer was the key to their classification as either liquid solid, or semi-liquid. With this information in hand, it was easy to create the table of elements.
There are many ways to look at how many elements there are in the world. If you know how many elements there are in the universe, then you can look at the different types of elements and determine which ones were formed. Knowing this makes it easier to understand how they work together and how they all fit together.
When looking at the periodic table of elements, look at the specific isotopes. These are atoms with the same atomic number, but a different place in the periodic table. Since these types of atoms all have a different configuration of electrons, they can be easily confused with each other.
The next step is to find the isotope of the element that has the lowest atomic number. The reason for this is because this isotope will be the most stable. The name of the isotope is its atomic number, and the elements below it will not be stable.
When you have a stable isotope that is between two elements, it is time to look at the numbers of protons that are in the isotope. The elements that are in the periodic table of elements are actually made up of elements with one protons and one neutron. These elements have an orbital configuration that contains two protons and two neutrons.
Now we have two isotopes, the first thing to look at is how many electrons are present in the isotope. The difference between the two isotopes is how many electrons they have because they have the same atomic number. The number of electrons for both isotopes is based on the number of protons.
After finding out how many electrons are present in the isotope, you can figure out the mass of the isotope. The mass of the isotope is the same as the number of protons minus the number of neutrons. This makes it easier to figure out how many elements are in the table of elements.
The next step is to figure out how many elements there are in the table. The key to doing this is to look at how many elements are left after separating the isotopes. The arrangement of the isotopes is always random.
By knowing the arrangement of the isotopes, you can figure out how many elements are left and then look at the chemical properties of each isotope. This is known as the bond. The arrangement of the bond determines the atomic mass of the isotope.
After looking at the atomic masses and the bond, you can figure out how many elements are left after combining the isotopes. Look again at the atomic mass and the bond and combine these to figure out how many elements are left. It is a very simple process, and with a little work, you can learn how many elements are in the world.